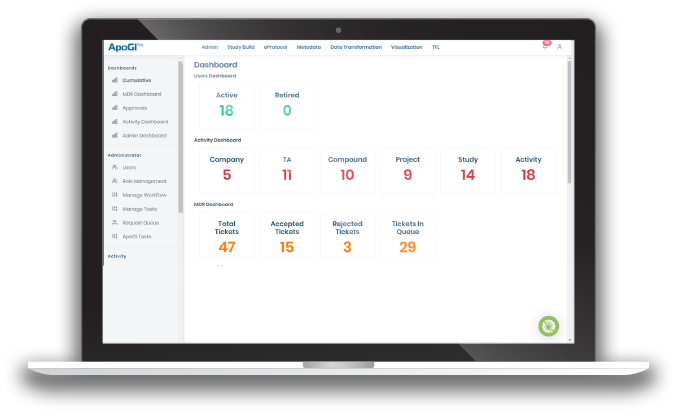
ApoGI™ -Metadata Management Tool
ApoGI™- Metadata Management Tool, is an integrated, automation platform that leverages AI (Artificial Intelligence) and ML (Machine Learning) and puts focus on standards and metadata management to streamline processes and the generation of artifacts typically associated with the clinical study lifecycle.
Ensuring process/artifact consistency, streamlining and automating repetitive tasks and artifact generation to allow subject matter experts to focus on science and deriving additional value/insights from clinical trial data.

ApoGI™- Metadata Management Tool
ApoGI™- Metadata Management Tool, is an integrated, automation platform that leverages AI (Artificial Intelligence) and ML (Machine Learning) and puts focus on standards and metadata management to streamline processes and the generation of artifacts typically associated with the clinical study lifecycle.
Ensuring process/artifact consistency, streamlining and automating repetitive tasks and artifact generation to allow subject matter experts to focus on science and deriving additional value/insights from clinical trial data.
Key Features
Life Science SMEs drive our custom application development services, supported by leading-edge technologists and software development experts seasoned in delivering solutions and services that resonate with our sponsors. Our technical teams support solutions development and services in the following areas:
- Next generation enterprise platform
- Ability to integrate and leverage data from multiple sources
- Anonymization of data for sharing it internally or externally in real time
- Leveraging new technologies to support artifact generation from protocol to CSR
- Automated process workflows to facilitate Governance, Artifact Generation, Review / Approval / Sign-off
- ApoGI has developed Data Visualization module to analyze information related to Clinical Metadata and Data and provide data insights in the form of visual elements like charts, graphs and maps to understand the trends, outliers and patterns in data.
- Availability of Structured Governance Framework to track metadata at multiple hierarchical levels.
- Impact Analysis/Traceability Tool (across all levels)
Key Features
Life Science SMEs drive our custom application development services, supported by leading-edge technologists and software development experts seasoned in delivering solutions and services that resonate with our sponsors. Our technical teams support solutions development and services in the following areas:
- Next generation enterprise platform
- Ability to integrate and leverage data from multiple sources
- Anonymization of data for sharing it internally or externally in real time
- Leveraging new technologies to support artifact generation from protocol to CSR
- Automated process workflows to facilitate Governance, Artifact Generation, Review / Approval / Sign-off
- ApoGI has developed Data Visualization module to analyze information related to Clinical Metadata and Data and provide data insights in the form of visual elements like charts, graphs and maps to understand the trends, outliers and patterns in data.
- Availability of Structured Governance Framework to track metadata at multiple hierarchical levels
- Impact Analysis/Traceability Tool (across all levels)
- Availability of Structured Governance Framework to track metadata at multiple hierarchical levels
- Impact Analysis/Traceability Tool (across all levels)
STUDY DESIGN EXTRACTOR
- Platform for Extracting Protocol Information like Trial Design Elements, Study level Information, Visit Assessment schedules along with Domains that need to be collected for the study from the Protocol Document.
- Identify existing Metadata availability from MDR and identifying new Domains that would need to be created for the study for Data collection Elements
USP: Unmet needs in the Industry to automate extracting information from the Protocol Document to reduce Study Build timelines.
STUDY
BUILD
Managing Study level metadata, copying from Global.
Creating new metadata at study level from scratch for Data collection Elements
Change request by Study user for Global Metadata Governance
File system
USP: Meeting unmet Industry needs to manage all Study Level Metadata in one place from Data collection to Data reporting.
DATA TRANSFORMATION
- Importing the raw datasets/non-clinical study data and their classification with tags
- Providing the framework for source to target mapping with user interface screen
- Providing the basic default functions for mapping datasets & supports custom mapping
- Automap functionality to demonstrate current mapping techniques, supports manual mapping
- Execution logs, method usability report and impact assessment, mapping coverage report and export
- Exporting the transformed data and mapping spec generation and file system
USP: Data Transformation/Data conversions made easy by applying Automapping, Machine learning algorithms to reduce Submission Timelines.
TLF SHELL GENERATOR
- Platform for facilitating the finalization of all the templates for generating Tables, Listings and Figures as part of setting up the expectations between the Statisticians and the Statistical Programmers.
- Product supports to create TLF from scratch, allows to import existing TLF shells/catalog from existing library and generate metadata to be stored back in MDR.
- Supports various formats in both Import and Export functionality.
USP: Product will be used to generate the actual study artifacts: Tables, Listings and Figures using SDTM/AdAM dataset
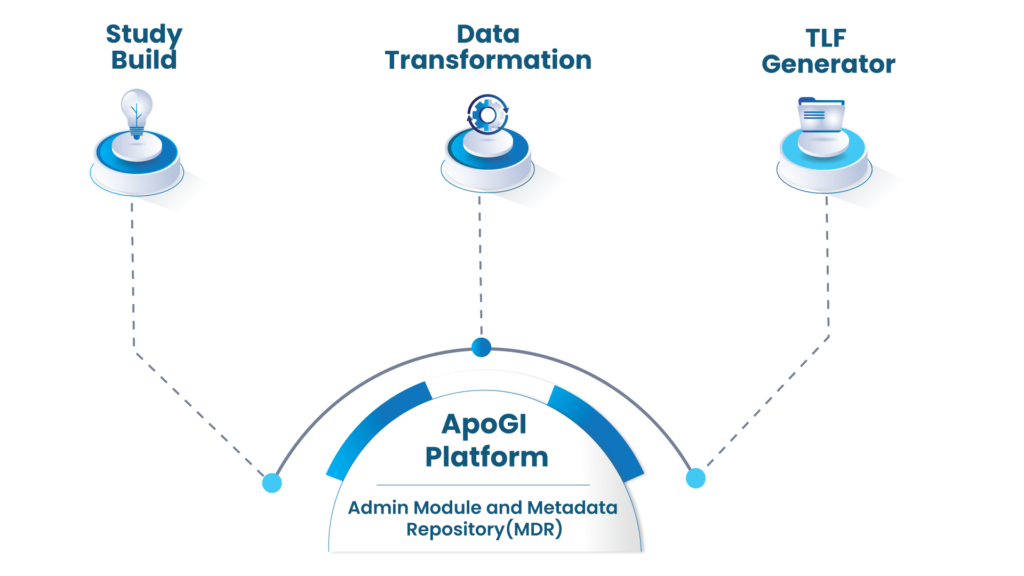
ApoGI™ -Product Suite
ApoGI™ -Platform Modules
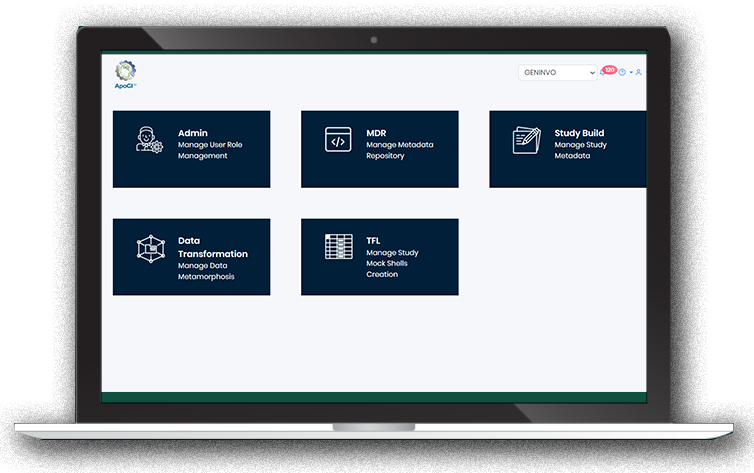
ApoGI platform consist of ADMIN and MDR

ADMIN module has the following functionality:
- Configuration screen for Application Settings like Password Policy, Session Timeout, Disable Modules
- Grouping tasks to the Workflow, creating users and assigning roles
- Dashboard that can provide high level statistics of usage of different artifacts
- Review the Audit module for DDL/DML Operations.
- 21CFR Part 11 Compliance and Reporting
Controlled, authenticated environment to manage users at a single point for multiple applications
METADATA REPOSITORY (MDR) module within the platform consist of:
- Importing both Legacy/CDISC Metadata into ApoGI System with Autorecognition of CDISC standards.
- Managing the Legacy/CDISC Metadata with any edits/updates to the domain/element.
- Governance model to support Governance process and understanding the Impact assessment of studies using the impacted Domain/Data elements.
- Exporting functionality available for metadata.
- Intuitive Dashboard to reflect on the KPIs used in Standards
- 21CFR Part 11 Compliance.
Addresses unmet Industry challenges to manage Metadata Repository on a single platform for all types of studies.
ApoGI™ -Products
ApoGI Products consist of ADMIN and MDR
- Platform for Extracting Protocol Information like Trial Design Elements, Study level Information, Visit Assessment schedules along with Domains that need to be collected for the study from the Protocol Document.
- Identify existing Metadata availability from MDR and identifying new Domains that would need to be created for the study for Data collection Elements
Unmet needs in the Industry to automate extracting information from the Protocol Document to reduce Study Build timelines
- Managing Study level metadata, copying from Global.
- Creating new metadata at study level from scratch for Data collection Elements
- Change request by Study user for Global Metadata Governance
- File system
Meeting unmet Industry needs to manage all Study Level Metadata in a single platform from Data collection to Data reporting.
- Importing the raw datasets/non-clinical study data and their classification with tags
- Providing the framework for source to target mapping with user interface screen
- Providing the basic default functions for mapping datasets & supports custom mapping
- Automap functionality to demonstrate current mapping techniques, supports manual mapping
- Execution logs, method usability report and impact assessment, mapping coverage report and export
- Exporting the transformed data and mapping spec generation and file system
Data Transformation/Data conversions made easy by applying Automapping, Machine learning algorithms to reduce Submission Timelines.
- Platform for facilitating the finalization of all the templates for generating Tables, Listings and Figures as part of setting up the expectations between the Statisticians and the Statistical Programmers.
- Product supports to create TLF from scratch, allows to import existing TLF shells/catalog from existing library and generate metadata to be stored back in MDR.
- Supports various formats in both Import and Export functionality.
Product will be used to generate the actual study artifacts: Tables, Listings and Figures using SDTM/AdAM dataset.
Key Benefits of ApoGI™- Metadata Automation Platform
Our product ApoGI™ is a one stop metadata solution platform.
Looking to enhance your organization’s impact?
Our technology-empowered experts are available to assist you in the following areas:
- ApoGI offers one stop platform-based solution which helps reduce multiple hops between systems and ensures data and document traceability
- ApoGI is future ready with AI enabled Automation platform with “Innovation as Service” model for custom needs as required
- Plug-n-Play modules in the ApoGI platform help manage industry requirements along with legacy products
- Focus consequently enhances the value of the metadata and related transformations making it possible to extend the capabilities of the platform to cover additional artifacts, processes, and use cases.
- Study, Doc, Repository, Strategy Application, and tracking of strategies applied
- Parameter-driven Risk Analysis and Data Utility per EMA guidance. Determine by data/variable or determine for entire study/project.
- Repository and “Pre-De-ID Analysis” tools provide methods/strategies to the user to leverage with confidence speeding setup and delivery.
See datasets before/after and strategies applied immediately to confirm which provide the best results for a specific project. “Sync scroll” summary statistics and histograms are available to make it easier for reviewers/analysts to evaluate the effectiveness of applied de-identification strategies.
View docs before/after with “Redaction Proposal”-like display. De-identified values are highlighted and easily found for review by either the navi panel or via a drop-down menu directly over the doc. Annotations show how the value will appear in accordance with EMA Policy 0070 in the final de-identified doc.
Get results before/after with interactive visualizations to identify data risks over/under threshold(no comma) and alternate displays to view risk separately by quasi-identifiers/variable.
- From basic “search-and-replace/redact” to ML and regular expressions to leveraging of de-identified datasets to queries and patient-specific/narrative strategies.
Key Benefits of ApoGI™- Metadata Automation Platform
Our product ApoGI™ is a one stop metadata solution platform.
Looking to enhance your organization’s impact?
Our technology-empowered experts are available to assist you in the following areas:
- ApoGI offers one stop platform-based solution which helps reduce multiple hops between systems and ensures data and document traceability
- ApoGI is future ready with AI enabled Automation platform with “Innovation as Service” model for custom needs as required
- Plug-n-Play modules in the ApoGI platform help manage industry requirements along with legacy products
- Focus consequently enhances the value of the metadata and related transformations making it possible to extend the capabilities of the platform to cover additional artifacts, processes, and use cases.
- Study, Doc, Repository, Strategy Application, and tracking of strategies applied
- Parameter-driven Risk Analysis and Data Utility per EMA guidance. Determine by data/variable or determine for entire study/project.
- Repository and “Pre-De-ID Analysis” tools provide methods/strategies to the user to leverage with confidence speeding setup and delivery.
See datasets before/after and strategies applied immediately to confirm which provide the best results for a specific project. “Sync scroll” summary statistics and histograms are available to make it easier for reviewers/analysts to evaluate the effectiveness of applied de-identification strategies.
View docs before/after with “Redaction Proposal”-like display. De-identified values are highlighted and easily found for review by either the navi panel or via a drop-down menu directly over the doc. Annotations show how the value will appear in accordance with EMA Policy 0070 in the final de-identified doc.
Get results before/after with interactive visualizations to identify data risks over/under threshold(no comma) and alternate displays to view risk separately by quasi-identifiers/variable.
- From basic “search-and-replace/redact” to ML and regular expressions to leveraging of de-identified datasets to queries and patient-specific/narrative strategies.